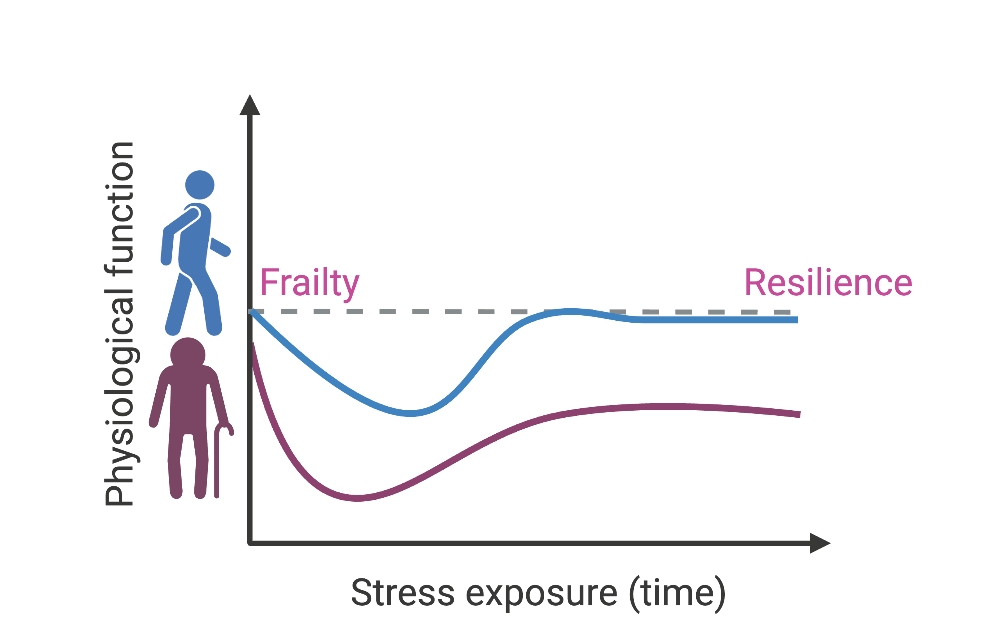
A few signs of old age are universal. As the years tick by, our hair becomes a little greyer, joints a little creakier, vision a little more blurry. Whether we can stay healthy in our golden years, however, is a bit more of a wild card. As each of us grows older, our bodies can change in a variety of unpredictable ways: some may still play weekly games of tennis with vigor, while others can be virtually bedridden. So why is it that even two people with seemingly identitcal genes can have such vastly different outcomes? And what is steering those differences?
As it turns out, trillions of bacteria living in our intestines—a group that’s collectively called our “gut microbiome”—may play an important role. The various species of bacteria present in the gut can change dramatically as we age, and at the same time, our metabolism, the sum total of all the biochemical reactions that take place in the human body, can shift as well.
This phenomenon could directly influence our wellbeing in our golden years, says Molecular Biologist Karthi Chellappa. As a new researcher at Brown’s Center on the Biology of Aging, she studies the interactions between our bodies and the microbes inside it, and is working to figure out some of the precise ways that they create and share energy with each other.
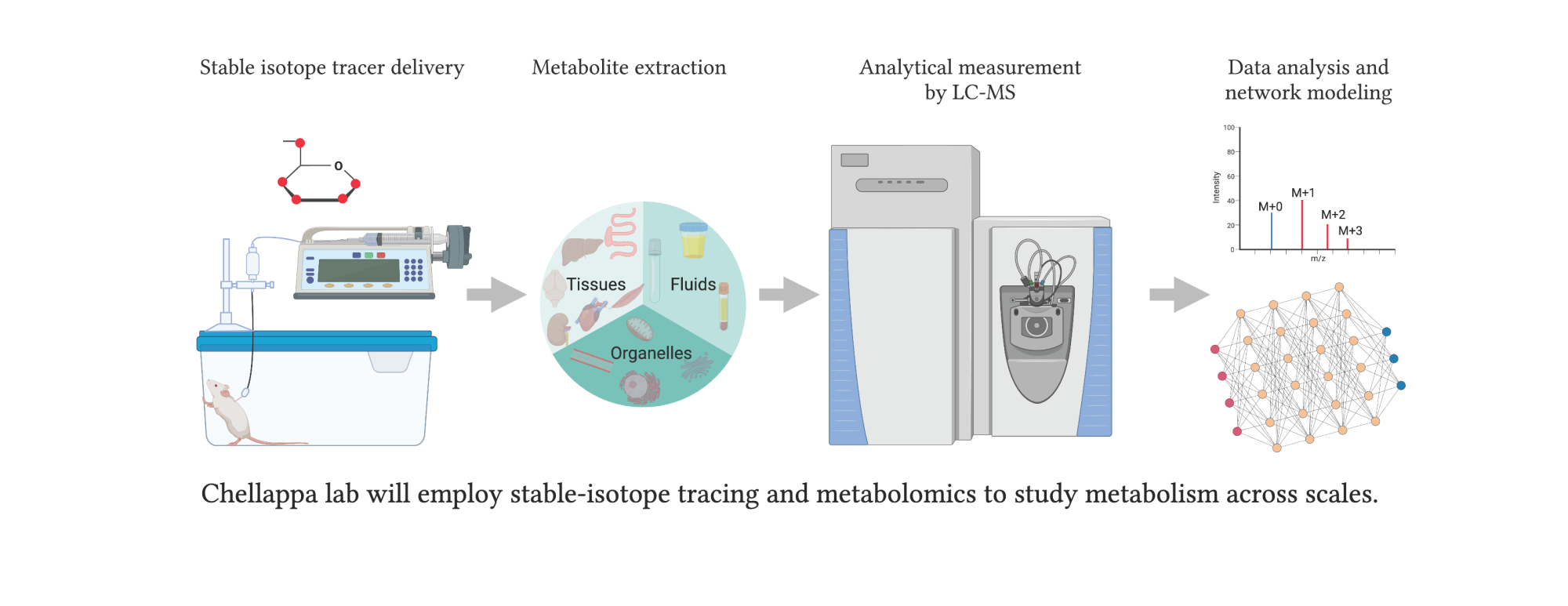
Chellappa’s work focuses on a substance in the human body called Nicotinamide Adenine Dinucleotide, or NAD, a ubiquitous molecule that is critical for most of our cells’ survival. Without it, she says, the basic chemical reactions that create energy in those cells—their metabolism—would grind to a halt, with catastrophic results.
Luckily, humans can make plenty of this key molecule. When we eat, our livers convert tryptophan, a common amino acid in most foods, into NAD, which is then used to create energy as cells break the NAD down into a precursor molecule called nicotinamide. This byproduct is distributed throughout the body, where it can salvaged to feed other cells, which in turn recycle much of it back into NAD as they create energy. Round and round it goes.
In the gut microbiome, this process works somewhat differently. Microbes can use the fiber we eat to make their own NAD directly—but Chellappa found that they can also take advantage of the nicotinamide that our cells generate, using it as one of their primary energy sources.
Gut microbes are considerate guests, however. In addition to using our body’s metabolic byproducts for survival , they also give back, cranking out molecules like nicotinic acid (also called Vitamin B3 or niacin), which our cells can use to make NAD. In this way, both our microbiome and our bodies collectively fuel each other.
“Before now, the common thinking was that microbes were largely self-functioning; that they didn’t need us to survive,” Chellappa says. “We’re learning that it’s really a symbiotic relationship. We didn’t understand that in the past.”
In her lab, she adds, she is trying to understand the basic links between microbial NAD and host metabolism: namely, how the microbiome’s metabolism affects our own cells and vice versa.
The Seeds of an Idea
Although she’s now steeped in the complex world of cellular biochemistry, Chellappa didn’t originally expect to end up there. Growing up in India, her real passion was nurturing plants. As she earned an undergraduate degree in agriculture and a master’s in horticulture, her focus gradually shifted. Instead of studying entire crops, she began to focus on the inner workings of plant cells, and later, the cells of animals.
“I realized I was most interested in the cellular processes that were going on, not the organism itself,” she says. “It was fascinating to me that in all these different species, the information in their genome wasn’t enough to drive the way their bodies worked. Environmental factors had a major impact as well.”
In that sense, our own bodies share a lot in common with even the lowliest weed. Most plants have a complex co-dependent relationship with microbes, fungi, and soil in order to thrive. Humans are no different. Without our microbiome, we’d be unable to absorb many nutrients, our immune system would suffer, and we’d generally be pretty miserable. In short, we need our microbes as much as they need us.
Chellappa wants to understand how this complex relationship shifts as we grow older, and how that change manifests itself in our overall health. In old age, she says, something in the microbiome’s normal metabolism transforms, altering the metabolites it provides our body. There could be a bunch of reasons for this shift—lowered physical activity, chronic stress, inflammation, and increased medications are just a few—but the real answer to this riddle will require a better understanding of the basic ways that cells create energy.
“NAD is one of the world's most studied molecules for more than a century. We know that NAD metabolism changes as we age, especially during disease states. But we still don’t know why,” she says. “Does this start with changes in the microbiome? With changes in our own cells? I think in the next five years, we’ll see an explosion in our field as we unravel those questions.”